Molybdenum cofactor deficiency: Molecular basis, diagnosis and therapy
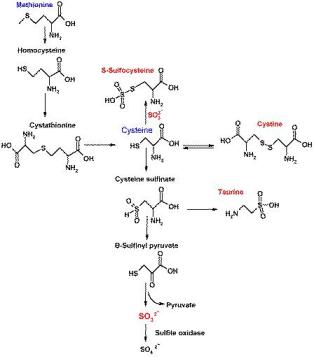
Human molybdenum cofactor (MCD) deficiency is a pleiotropic autosomal recessive genetic disorder characterized by the loss of the molybdenum-dependent enzymes sulfite oxidase, xanthine oxidoreductase and aldehyde oxidase. Patients are characterized by progressive neurological damage leading in most cases to early childhood death, mainly caused by the deficiency of sulfite oxidase that protects the organism, in particular the brain, from elevated levels of toxic sulfite and provides sulfae as metabolite. Sulfite is derived from the degradation of cysteine, methionine, and sulfatides. So far, disease-causing mutations have been identified in three of the four known Moco-synthetic human genes: MOCS1, MOCS2, and GEPH.
Projects
We are interested in the diagnosis, molecular basis and therapy of molybdenum cofactor deficiency.
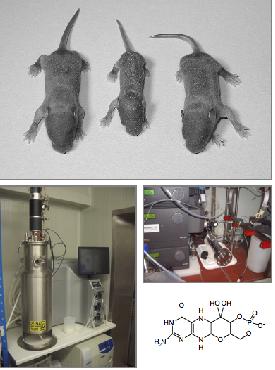
- An animal model, generated by our collaborator Prof. J. Reiss (University of Goettingen), resembles the phenotype of human MCD patients. Homozygous MOCS1 deficient mice die within 11 after birth due to the loss of all Mo enzymes.
- The first intermediate of Moco synthesis, cyclic pyranpterin monophophate (cPMP) was fermented in E. coli, purified, structurally characterized and successfully used to cure MCD in MOCS1-deficient mice
- Currently we are establishing a cPMP fermentation and production procedure for a clinical trial to treat MCD
- The molecular basis of MCD is studied by proteomics and metabolomics
Funding

- BMBF (Bioprofil) (2005-2008)
- DFG Schw 759/6-1 (2004-2006)
Tools
- fermentation of cPMP in E. coli
- preparative HPLC purification of cPMP and stabilization
- HPLC analysis of pterin derivatives, nucleotides and related metabolites
- treatment of MOCS1 knockout mice with cPMP
- enzyme substitution therapy
Diagnostics
- determination of cPMP and molybdopterin/Moco content by HPLC analysis and enzyme reconstitution assays
- determination of Mo enzyme activities (sulfite oxidase, xanthine dehydrogenase)
- mutation analysis can be requested from Prof. Dr. Jochen Reiss (University Goettingen)
Collaboration

- Prof. Dr. Georg Hanisch
xxxx - Prof. Dr. Jochen Reiss
Institute for Human Genetics, University of Goettingen, Germany - PD Dr. Joern-Oliver Sass
Kinderklinik, University of Freiburg, Germany
Project-relevant publications
- Reiss, J., Bonin, M., Schwegler, H., Sass, J.O., Garattini, E., Wagner, S., Lee, H.-J., Engel, W., Riess, O., Schwarz, G.* 2004
The pathogenesis of molybdenum cofactor deficiency and its delay by maternal clearance.
Mol. Gen. Metabol. 85, 12-20 - Schwarz, G., Santamaria-Araujo, J. A., Wolf, S., Lee, H. J., Adham, I. M., Grone, H. J., Schwegler, H., Sass, J. O., Otte, T., Hänzelmann, P., Mendel, R. R., Engel, W., Reiss, J. 2004.
Rescue of lethal molybdenum cofactor deficiency by a biosynthetic precursor from Escherichia coli.
Hum. Mol. Gen. 13: 1249-55 - Santamaria Araujo, J.A., Fischer, B. Otte, T., Nimtz, M, Wray, V, Mendel, R.R. and Schwarz, G.* 2004
The structure of the sulfur- and molybdenum-free pyranopterin precursor of the molybdenum cofactor.
J. Biol. Chem. 279: 15994-15999 - Lee, H.-J., Adham, I.M., Schwarz, G., Kneussel, M., Sass, J.-O., Engel, W., and Reiss, J. 2002.
Molybdenum cofactor-deficient mice resemble the phenotype of human patients.
Hum. Mol. Gen. 11:3309-3317. - Reiss, J., Gross-Hardt, S., Christensen, E., Schmidt, P., Mendel, R.R., and Schwarz, G.* 2001.
A mutation in the gene for the neurotransmitter receptor-clustering protein gephyrin causes a novel form of molybdenum cofactor deficiency.
Am. J. Hum. Genet. 68:208-213.