Postsynaptic scaffolds at inhibitory synapses
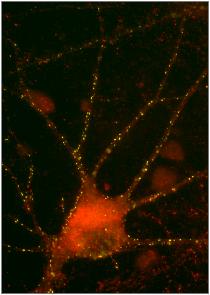
Glycine (GlyR) and gamma amino butyric acid type A receptors (GABAAR) are inhibitory neuro-receptors that belong to the superfamily of ligand-gated pentameric ion channels (Cys-loop family). Localization of these receptors in time and space is crucial for efficient synaptic transmission and the precise regulation of many neuronal functions. The multifunctional protein gephyrin plays a critical role in organizing postsynaptic structures at inhibitory synapses. It has been postulated to form a sub-membranous scaffold by trimerization of its N-terminal G-domain and dimerization of its C-terminal E-domain. The molecular mechanism of gephyrin oligomerization, trafficking and receptor organization is still poorly understood. GABAA receptors are primary mediators of inhibitory neurotransmission in the adult brain. They are involved in a number of central nervous system (CNS) diseases, including epilepsy, sleep disturbances, alcoholism, chronic pain, schizophrenia, and others.
Projects
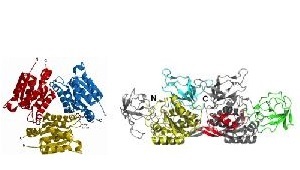
- Molecular basis of postsynaptic clustering and functional diversity of gephyrin
We have determined crystal structures of the individual gephyrin domains and characterized the interactions with the glycine receptor and other signaling and cytoskeletal proteins. Alternative splicing and tissue-specific expression of gephyrin has been proposed to contribute to gephyrin´s multiple functions, which is currently investigated
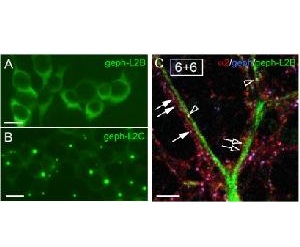
- Clustering mechanism of GABAA receptors
In addition to glycine receptors also major subtypes of GABAA receptors are organized in a gephyrin-dependent manner. In contrast to the well-known interaction between gephyrin and the ?-subunit of the glycine receptor it is not known how gephyrin clusters interact with GABAARs and which subtype of the large variety of GABAARs is organized by gephyrin, which is studied in this project. Furthermore, we investigate other GABAA receptor-associated cytoplasmic proteins
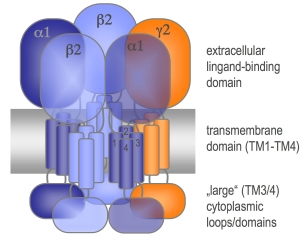
- Structure and function of ligand-gated ion channels
Different models for glycine and GABAA receptor structures have been published an experimentally determined structure of any receptor of the pentameric ligandgate ion channel family is still missing. As GABAARs present important pharmacological targets and show a large variety and complexity we will study the biochemical and structural properties of these receptors
Funding
- DFG Schw 759/2-4 (2005-2008)
- DFG Schw 759/8-1 (2007-2010)
- ZMMK (2008-2010)
Tools
- standard tools in molecular biology and biochemistry (PCR, molecular cloning, Western blots, enzyme assays, etc.)
- recombinant protein expression in E. coli, yeast, and animal cells (incl. fermentation)
- protein purification by affinity chromatography (IMAC, GST, intein, strep, etc.)
- biophysical methods (UV/vis spectroscopy, lights scattering, etc.)
- protein interaction studies and ligand binding (isothermal titration calorimentry, co-sedimentation)
- protein crystallography and structural biology
- GFP-fusion, expression in animal cells and colocalizations
Collaboration
- Prof. Dr. Jean-Marc Fritschy
Institute of Pharmacology, University Zurich, Switzerland - Prof. Dr. Jochen C. Meier
Max-Delbrück Center for Molecular Medicine, Berlin-Buch, Germany - Prof. Dr. Hermann Schindelin
Rudoph-Virchow-Center for Experimental Medicine, University of Wuerzburg, Germany
Project-relevant publications
- Lardi-Studler B, Smolinsky B, Petitjean CM, Koenig F, Sidler C, Meier JC, Fritschy JM, Schwarz G. 2007.
Vertebrate-specific sequences in the gephyrin E-domain regulate cytosolic aggregation and postsynaptic clustering.
J Cell Sci. 120:1371-82 - Xiang, S., Nassar, N., Kirsch, J., Winking, J., Schwarz, G., and Schindelin, H. 2006.
Insights into Collybistin Function from the Crystal Structure of the Cdc42-Collybistin II Complex.
J Mol Biol 359, 35-46 - Kim, E. Y., Schrader, N., Smolinsky, B., Bedet, C., Vannier, C., Schwarz, G., and Schindelin, H. 2006.
Deciphering the structural framework of glycine receptor anchoring by gephyrin.
EMBO J 25:1385-1395 - Schrader, N., Kim, E.Y., Winking, J., Paulukat, J., Schindelin, H., and Schwarz, G.* 2004.
Biochemical characterization of the high affinity binding between the glycine receptor and gephyrin.
J. Biol. Chem. 279: 18733-18741 - Jockusch, B.M., Rothkegel, M., and Schwarz, G. 2004.
Linking the Synapse to the Cytoskeleton: a Breath-taking Role for Microfilaments
Neuroreports 15: 1535-38 - Giesemann, T., Schwarz, G., Navrotzki, R., Berhörster, K., Rothkegel, M., Schlüter, K., Schrader, N., Schindelin, H., Mendel, R.R., Kirsch, J., and Jockusch, B. 2003.
Profilin and Mena link the postsynaptic scaffold protein gephyrin to the microfilament system.
J. Neurosci. 23: 8330-8339 - Schwarz, G., Schrader, N., Mendel, R.R., Hecht, H.J., and Schindelin, H. 2001.
Crystal Structures of Human Gephyrin and Plant Cnx1 G Domains: Comparative Analysis and Functional Implications.
J. Mol. Biol. 312:405-418. - Reiss, J., Gross-Hardt, S., Christensen, E., Schmidt, P., Mendel, R.R., and Schwarz, G.* 2001.
A mutation in the gene for the neurotransmitter receptor-clustering protein gephyrin causes a novel form of molybdenum cofactor deficiency.
Am. J. Hum. Genet. 68:208-213. - Stallmeyer, B., Schwarz, G., Schulze, J., Nerlich, A., Reiss, J., Kirsch, J., and Mendel, R.R. 1999.
The neurotransmitter receptor-anchoring protein gephyrin reconstitutes molybdenum cofactor biosynthesis in bacteria, plants, and mammalian cells.
Proc. Natl. Acad. Sci. U. S. A. 96:1333-1338.